The Sputnik V vaccine is not accepted as valid proof of immunity in the United Kingdom. The proposed attributes and criteria provide considerations for the evaluation and prioritization of COVID-19 candidate vaccines to be considered for further development by WHO.

Russia Sees No Hurdles For Who Approval Of Sputnik V Covid 19 Vaccine The Financial Express
WHO Suspends Approval Process For Russias Sputnik Vaccine Official Says.
Sputnik vaccine who approval. Russias Sputnik V Covid-19 vaccine is finally set to be approved by the WHO after the countrys health minister said only minor administrative procedures remain to be finalized following a meeting with the UN agencys chief. By News Source Guyana on October 9 2021. The World Health Organization WHO has suspended the approval process for Russias Sputnik V COVID vaccine.
Sputnik V is now produced in about twenty countries and authorized for use in 70 countries. A regional WHO official said the manufacturing process of. By the end of February 2021 Sputnik V was approved by national regulators of Ghana Syria Kyrgyzstan Guiana Egypt Honduras Guatemala and Moldova.
12th October 2021 2328 IST Sputnik V COVID Vaccine May Get WHO Approval By Year End Says Chief Scientist Russias Sputnik V COVID-19 Vaccine might be approved by World Health Organization before the end of the year if Moscow signs the appropriate legal documents. September 16 2021 1237 GMT. WHO says approval process for Russias Sputnik V vaccine still on hold.
WHO-approved vaccines to be recognised for travel to the US. Sputnik still not approved. The UK has only recognized the four vaccines that have been approved by EMA AstraZeneca PfizerBioNTech Moderna Johnson Johnson.
Updated at 416 am. The Russian-made Sputnik V vaccine which has been administered to over 175000 persons in Guyana is still awaiting WHO approval and therefore persons administered with the vaccine will not be allowed to travel to the US. This was confirmed by the WHO director-general Murashko told reporters in GenevaMeanwhile the company which developed the Russian Sputnik V vaccine against coronavirus the Gamaleya Research Institute hopes that the drug will receive necessary approval from the World Health Organization WHO by the end of the yearI believe it will happen in the coming months -.
Consequently all persons who have. 21 to add RDIFs statement. On the official page of Sputnik V on Twitter it saysthat the vaccine prequalification process is on track and in its final stages A group of WHO inspectors should soon visit Russia to carry out all the.
AstraZeneca Covishield AstraZeneca Vaxzevria and Moderna Takeda are also accepted. KAZINFORM - The World Health Organization WHO will decide on including the Russian Sputnik V COVID-19 vaccine in the Emergency Use Listing EUL after the last inspection in. A regional WHO official said one manufacturing plant for the vaccine.
The Sputnik V shot widely used in Russia and approved for use in over 70 countries is undergoing a review by the WHO and the European Medicines Agency EMA. It was however stated on 16th September 2021 that WHO has suspended the approval process of the Covid-19 vaccine Sputnik V due to. 104 On 30 April 2021 Turkey and Albania approved the use of Sputnik V vaccine for emergency use.
Initially produced in Russia Sputnik V uses a weakened virus to deliver small parts of a pathogen and stimulate an immune response. Russias Sputnik V COVID vaccine on hold pending missing data and legal procedures UN body says. Russia applied for emergency authorisation for the Sputnik V vaccine in February but it has not yet been approved by the WHO.
Under the WHO Emergency Use Listing EUL Procedure WHO has so far carried out 9 inspections of the Sputnik V vaccine clinical trial and manufacturing sites - 5 clinical trial sites were inspected jointly with EMA for Good Clinical Practices and 4 manufacturing sites were inspected for Good Manufacturing Practices 2 inspected jointly with EMA. On 27 April 2021 Bangladesh approved the use of Sputnik V vaccine for emergency use. Reuters - The World Health Organization said on Wednesday the Emergency.
By RFERLs Russian Service. The Sputnik V Gam-COVID-Vac is an adenoviral-based two-part vaccine against the SARS-CoV-2 coronavirus. WHO says approval for Russias Sputnik V vaccine still on hold.
Japan approved the vaccine for emergency use in May 2021 but doesnt plan to use them immediately because of rare cases of a blood clotting disorder reported overseas. The World Health Organization has resumed the approval procedure for the Russian Sputnik V coronavirus vaccine for emergency use should from the register of the organization. Later Japan started to use the vaccine for people aged 40 or over to mitigate the surge of the Delta variant in August.
GENEVA Sputnik - The World Health Organisation WHO could approve Russias Sputnik V COVID-19 vaccine by the end of the year if Moscow signs the necessary legal documents in the coming days WHO Chief Scientist Soumya Swaminathan said in an interview with Sputnik. On similar lines Sputnik V expects its approval within the next two months as well as there have been no critical remarks for now at all. The European Unions drug regulator will not approve Russias Sputnik V coronavirus vaccine until at least the first months of 2022.
The Russian COVID-19 vaccine Sputnik V is expected to get World Health Organizations WHO approval in September-October this year said Kirill. The target audience includes vaccine scientists product developers manufacturers regulators and funding agencies.

Who Suspends Approval Process For Russia S Sputnik Vaccine Official Says

Israel Joins 100 Other Countries In Accepting The Sputnik V Vaccine
Sputnik Light In India Dr Reddy S Denied Approval To Conduct Phase 3 Trials
Brazilian Approval Of Sputnik V Vaccine Delayed By Missing Data Reuters
Who Says Approval Process For Russia S Sputnik V Vaccine Still On Hold Top News Us News

Coronavirus Vaccine Limited Sputnik V Doses By April End In India 850 Million Doses Annually

Fact Check How Effective Is The Sputnik V Coronavirus Vaccine Science In Depth Reporting On Science And Technology Dw 15 04 2021

U S Pushed Brazil To Reject Russia S Sputnik V Vaccine Hhs Report Says The Washington Post

Dr Reddy S Denied Approval For Phase 3 Trials Of Sputnik Light Vaccine Business Standard News
Who Says Near To Solving Issues On Russia S Sputnik V Vaccine Reuters
Russia Presses Bid For Who Approval For Sputnik V Vaccine World News Hindustan Times

Covid Hungary Fast Tracks Russian Vaccine With Eu Approval In The Works News Dw 21 01 2021

Uae Begins Trials Of Russia S Sputnik V Covid 19 Vaccine
Growing Evidence Suggests Russia S Sputnik V Covid Vaccine Is Safe And Very Effective But Questions About The Data Remain

Eu Postpones Approval Of Russian Sputnik V Vaccine Until Next Year Schengenvisainfo Com
Dr Reddy S Completes Sputnik Vaccine Phase 3 Trials The Hindu Businessline
Dr Reddy S Completes Sputnik Vaccine Phase 3 Trials The Hindu Businessline

Russia S Sputnik V Vaccine Has Not Yet Received Eu And Who Backing Drug Discovery And Development
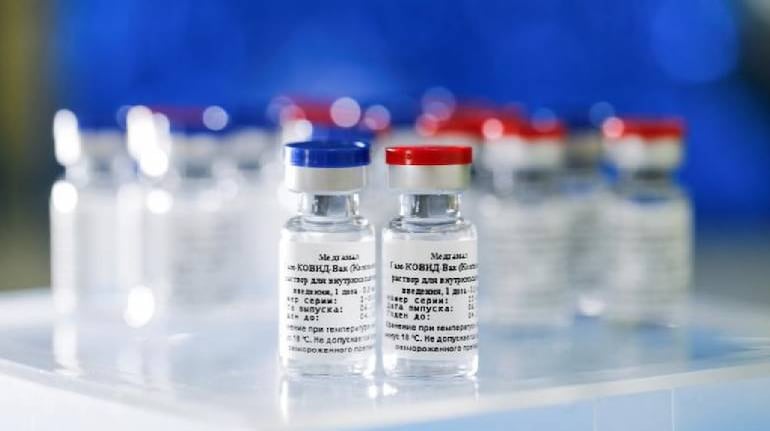
Russia Sees No Hurdles For Who Approval Of Sputnik V Vaccine

