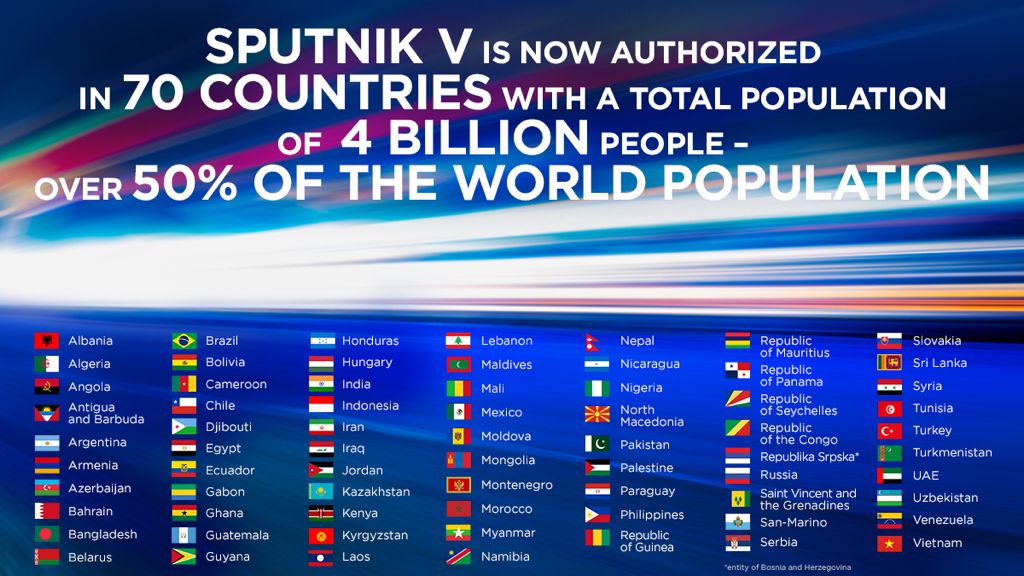
Гам-КОВИД-Вак is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in RussiaIt is the worlds first registered combination vector vaccine for the prevention of COVID-19 having been registered on 11 August 2020 by the Russian Ministry of Health. Total population of all countries where russian vaccine covid Sputnik V is approved for use now exceeds 4 billion people which is more half of the global population.

Commentary What Happened To Russia S Sputnik V Covid 19 Vaccine Cna
Sputnik V was granted an emergency use authorization the RDIF said.

Sputnik vaccine indonesia. Vaccine Trial Approval Tracker. As per the Russian Direct Investment Fund RDIF Sputnik V has been given emergency usage authorisation. Sputnik V Vaccine Description.
Спутник V or Gam-COVID-Vac Russian. Indonesia starts its vaccine rollout this week Russia has inoculated almost 1 million and the Vatican will jab everyone - including the Pope - this month. The UK has only recognized the four vaccines that have been approved by EMA AstraZeneca PfizerBioNTech Moderna Johnson Johnson.
Indonesia and Russia are finalizing a memorandum of understanding MoU on health cooperation to produce the vaccine. TEMPOCO Jakarta- Indonesian Foreign Affairs Minister Retno Marsudi on Tuesday announced that a team from the Indonesian Food and Drugs Monitoring Agency BPOM had paid a visit to the production facility of Sputnik V vaccine in Russia. The Sputnik V vaccine is not accepted as valid proof of immunity in the United Kingdom.
With this Indonesia has now become the worlds 70th nation to approve the Russian vaccine. Indonesia has approved Russias Sputnik V COVID-19 vaccine for emergency use in the Southeast Asian country Penny Lukito the head of the food and. Indonesia has become the 70th country in the world to register the Russian vaccine.
Rapid rollouts of Sinovac Sputnik. Sun 31 Oct 2021 0430 EDT. Lukito the head of the Food and Drug Supervisory Agency BPOM said the agencys reviews on Sputnik-V performance conclude that the vaccine satisfied internationally applicable vaccine quality.
Caught in vaccine limbo. Sputnik-V would follow China-made Sinovac and Sinopharm vaccines as well as American Moderna Pfizer and Anglo-American AstraZeneca vaccines which are already available in Indonesia. It consists of the first dose of the Sputnik V vaccine which is based on the Ad26 vector and it can be stored at a.
Indonesias National Agency of Drug and Food Control announced on Wednesday that the Russian-made Sputnik V coronavirus vaccine. Indonesia has so far recorded over 4000000 COVID-19 cases. The Indonesian Food and Drug Supervisory Agency BPOM head Penny Lukito has visited Russia to see firsthand the Sputnik V vaccine production facility.
Amin said that Eijkman welcomes the partnership. Sputnik Light as a booster for other vaccines will be almost as effective against the Delta variant as Russias flagship two-shot Sputnik V vaccine RDIF which markets Sputnik V internationally. AstraZeneca Covishield AstraZeneca Vaxzevria and Moderna Takeda are also accepted.
Moscow August 25 2021 The Russian Direct Investment Fund RDIF Russias sovereign wealth fund announces the approval of the Russian Sputnik V vaccine against coronavirus in the Republic of Indonesia. 9 Vaccines Approved for Use in Indonesia. AFP Photo The World Health Organization WHO said on Thursday it was about to restart the process of approving Russias.
This article is reviewed regularly on a monthly basis by Wegos editorial team to ensure that the content is up to date accurate. Indonesia has approved Russias Sputnik V coronavirus vaccine for emergency use according to a statement published on the website of the countrys National Agency of. Total population of all countries where Sputnik.
Last Updated 29 October 2021. When Denis Ovchinnikov read the news this summer that his Russian Sputnik V vaccine would not be recognised in. Although Sputnik V was launched in July 2020 with Putin Administration claiming it to be worlds first COVID vaccine Sputnik V is yet to receive a WHO Emergency Use Listing EUL.
This vaccine is approved. WHO To Restart Sputnik Vaccine Analysis. Sputnik V was granted an emergency use authorization EUA in Indonesia the 70th country to register the Russian COVID vaccine.
Indonesia has become the 70th country to register the Russian vaccine. At present the vaccine is approved by 70 countries making it the second most approved coronavirus vaccine in. Indonesian Trade Minister Muhammad Lutfi said in an interview with Izvestia in June 2021 that Indonesia was going to grant emergency use authorization to the Russian vaccine.
A health worker shows a dose of the second component of the Sputnik V vaccine against COVID-19 at the National Sports Council in Asuncion on 18 August 2021. The Sputnik V vaccine works in a similar way to the AstraZeneca product. Consequently all persons who have.
ETHealthWorld August 26 2021 1027 IST. Sputnik V was granted an emergency use authorization EUA in Indonesia the 70th country to register the Russian COVID vaccine. Pjotr Sauer in Moscow.
She said that during the working visit of Russian Foreign Affairs Minister Sergei Lavrov to Jakarta on July 6. Read more Uttarakhand CM Pushkar Singh Dhami launches Sputnik V vaccine. Russias COVID-19 vaccine Sputnik V has been approved for use in dozens of countries and its also under review by the European Medicines Agency.
But the vaccine remains controversial. The Russian COVID-19 vaccine Sputnik V Gam-COVID-Vac is an adenoviral-based two-part vaccine against the SARS-CoV-2 coronavirusInitially produced in Russia Sputnik V uses a weakened virus to deliver small parts of a pathogen and stimulate an immune response. Anhui Zhifei Longcom ZF2001.
By now it has been approved in 70 different countries and. Indonesia has authorised Russias Sputnik V COVID-19 vaccine for emergency use in the country. Sputnik V was granted an emergency use authorization.
Updated 15 September 2021 The Russian Sputnik vaccine has been one of the front-runners from the start of the COVID-19 vaccine race. A man receives a dose of the coronavirus disease COVID-19 vaccine at a drive thru vaccination station during the vaccination program in Karawang West Java province Indonesia August 24 2021 in this photo taken by Antara FotoM Ibnu Chazarvia Reuters JAKARTA Reuters - Indonesia.

Https Static Dw Com Image 56396650 401 Jpg

Https Static Dw Com Image 57716641 101 Jpg
Sputnik V Sputnikvaccine טוויטר
Indonesia Clears 7th Vaccine For Use To Bolster Virus Fight
Growing Evidence Suggests Russia S Sputnik V Covid Vaccine Is Safe And Very Effective But Questions About The Data Remain
Russia Clears Single Dose Sputnik Light Vaccine For Use
Chinese Firm To Produce 60m Doses Of Sputnik V Vaccine

India Among 20 Countries Interested In Obtaining Russian Covid Vaccine Sputnik V India News
Put Idle Capacity To Work Now Making Vaccines Says Wto Head Reuters
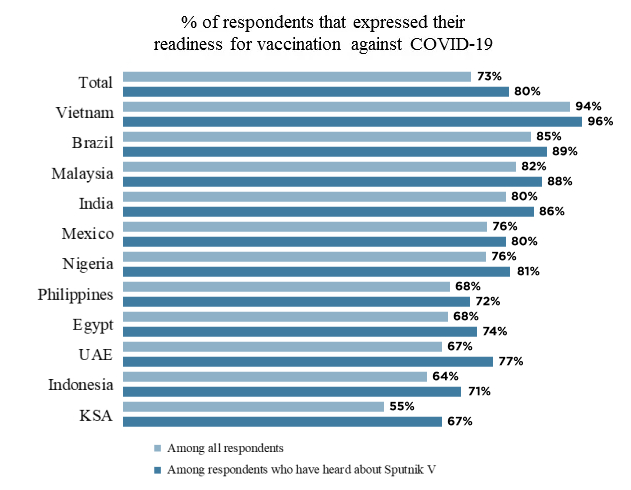
Russia Is Considered A Highly Trusted Vaccine Manufacturer And Almost Half Of Respondents In 11 Countries Are Aware Of Sputnik V A Yougov Poll Shows Russian Direct Investment Fund

This Asian Country Plans To Give Covid Vaccines To Younger People First
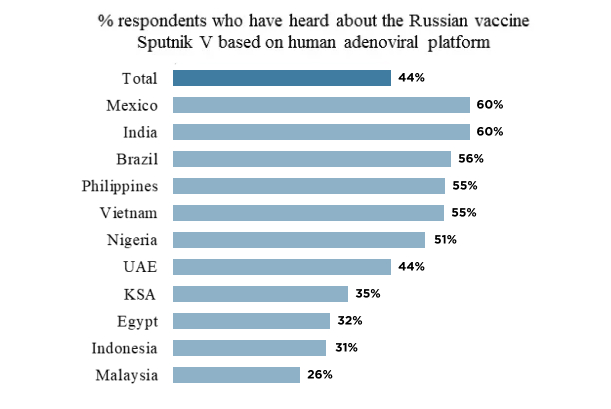
Russia Is Considered A Highly Trusted Vaccine Manufacturer And Almost Half Of Respondents In 11 Countries Are Aware Of Sputnik V A Yougov Poll Shows Russian Direct Investment Fund
Russian Sputnik V Vaccine 95 Efficient Developer
Russia S Rdif Sputnik V Vaccine To Be Approved By 25 Countries Within Weeks Reuters Com
73 Countries Recognize Sputnik V As Valid Proof Of Vaccination For Travel Visaguide World

Indonesia Clears Seventh Vaccine For Use To Bolster Virus Fight Cooperates With China To Build Covid 19 Vaccine The Star
Argentina Requests Russia To Guarantee Vaccine Supply
Sputnik V Indonesia Approves Sputnik V For Emergency Use Health News Et Healthworld
Indonesia Approves Sputnik V Covid 19 Vaccine For Emergency Use Vestnik Kavkaza
